Company Biography
Deciphera is a biopharmaceutical company focused on discovering, developing and commercializing new medicines to improve the lives of people with cancer. We leverage our clinical-stage proprietary switch-control kinase inhibitor platform and expertise in kinase biology to develop a broad portfolio of innovative medicines. https://www.deciphera.com/
QINLOCK® is our first FDA-approved product for eligible advanced GIST patients who have received 3 prior TKIs, including imatinib. Please see Important Safety Information and full Prescribing Information for QINLOCK.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)
RIPRETINIB (QINLOCK) IS A CATEGORY 1 PREFERRED TREATMENT FOR 4TH-LINE ADVANCED GIST. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastrointestinal Stromal Tumors (GISTs) V.1.2021. ©National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Published October 30, 2020. Accessed October 30, 2020. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.

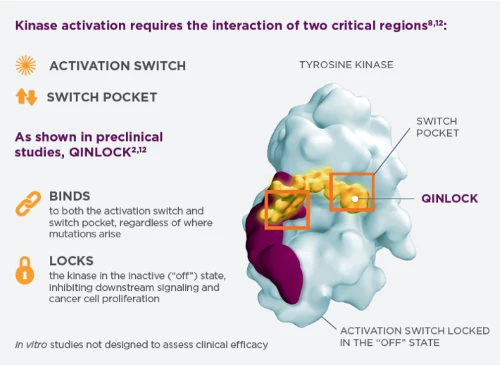
QINLOCK (ripretinib) Mechanism of Action
QINLOCK--engineered to block the drivers of resistance in advanced GIST. QINLOCK is the first and only switch-control kinase inhibitor that provided broad-spectrum inhibition of KIT and PDGFRa kinase signaling in vitro through a novel dual mechanism of action. As shown in preclinical studies, QINLOCK binds to both the activation switch and switch pocket of the KIT and PDGFRa kinase and locks the kinase in the inactive ("off") state, inhibiting downstream signaling and cancer cell proliferation. In preclinical studies, this dual mechanism provided broad spectrum inhibition of KIT and PDGFRa kinase activity, including multiple primary mutations, multiple secondary mutations, and wild type. In vitro studies not designed to assess clinical efficacy.
SELECT SAFETY INFORMATION. There are no contraindications for QINLOCK. Palmar-plantar erythrodysesthesia syndrome (PPES): In INVICTUS, Grade 1-2 PPES occurred in 21% of the 85 patients who received QINLOCK. PPES led to dose discontinuation in 1.2% of patients, dose interruption in 2.4% of patients, and dose reduction in 1.2% of patients. Based on severity, withhold QINLOCK and then resume at same or reduced dose. Please see additional safety information and full Prescribing Information.
Contact Information
Waltham, MA 02451
Team Members